TABLE OF CONTENTS
Report training
Ensure you have access to the TPP Learning Academy and then access the reporting course: https://training.tpp-uk.com//gp/reporting
Notes/Tips from the ICB Clinical Systems team
Completed the elearning and starting to build your first clinical reports? We've logged the following items as:
- they are common pitfalls
- or their importance for clinical safety and/or data protection
Admin/Support staff
While you can build/maintaint them, any reports that will result in clinical/care actions should have oversight and routine review by a suitably qualified clinician.
Sharing report extracts/data
If you are building reports to disclose to other services or for research purposes etc, we recommend you discuss each case with your Data Protection Officer. One of the considerations of these discussions will be if the disclosure is in scope for National Data Opt-Out (NDO) - historically called 'Type 2' opt outs.
Where relevant, use the Data Dissemination category in your report(s) to apply patient NDO decisions:

Code Clusters
Many groups of codes already have pre-made groupings; there are called Code Clusters in SystmOne and formally called 'Reference sets' (refsets) in SNOMED.
Much of the data that has an automated extract or target will have associated code clusters, such as QOF and Enhanced Services. For example (at time of writing):
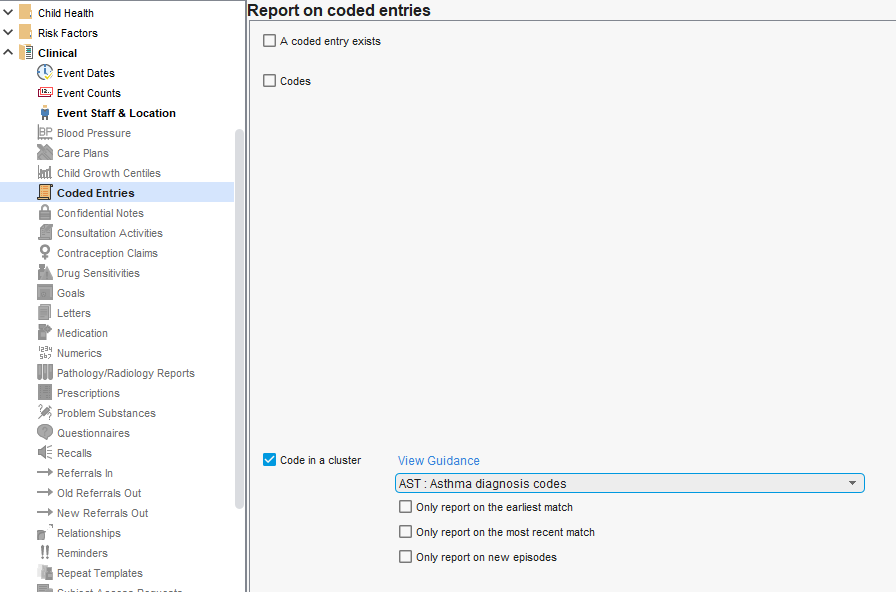
- AST: Asthma diagnosis codes
- NOCX: Codes that indicate complete removal of the cervix
- MALT: Codes for microalbuminuria testing
Using a Code cluster means you will match the national standards for that cluster rather than needing to select codes yourself.
Be aware that different data standards (and therefore, different code clusters) can exist for similar things. Which cluster you should use will depend on each case. For example:
- ALC: Alcohol consumption codes (a generic alcohol consumption code list)
- NCDALC: NCD Alcohol consumption codes (for the Network Contract Directed Enhanced Service)
- PHSMIALC: PHSMI Alcohol consumption codes (for the Physical Health checks for Severe Mental Illness programme)
Example of using Codes in a cluster (Asthma diagnosis codes selected) rather than manually defining a code list with the Codes option:
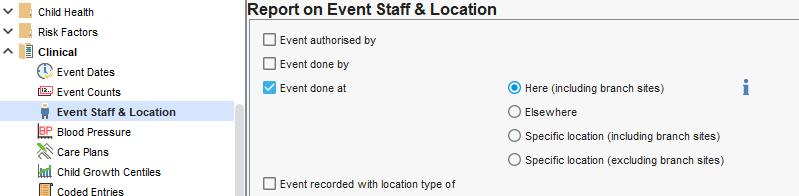
Event Location
SystmOne is a shared records solution. By default when creating a clinical report the system will consider all data available to your service, not only data entered or owned by your service. When creating reports for clinical need this is normal and recommended. e.g. If looking for patients not on the Hypertension register with a high blood pressure reading; it doesn't matter if your service or another recorded this.
Where identifying activity performed exclusively by your service, some of your reports may need to be restricted to events done "here":
Registration Status & Registration Type
Reports default to active registered patients. If you are looking for activity perfromed in a period (note just of those still registered) your service may need to included deceased and deducted patients.
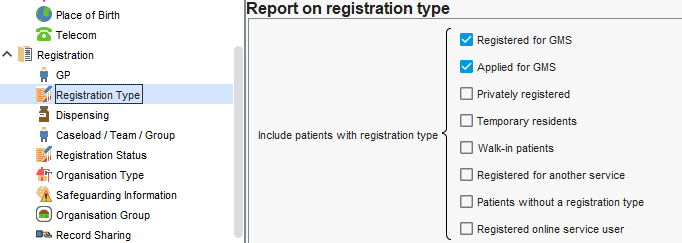
If joining reports, ensure all have this specified where needed.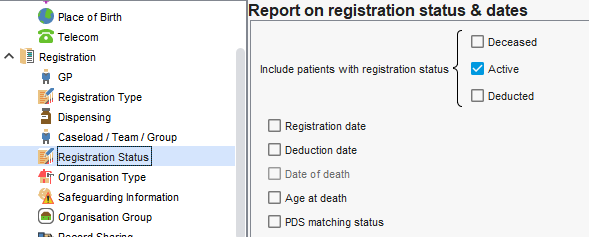
Similarly, your system will have a default inclusion of patient types that you may need to adjust depending on what your serivce istrying to identify. Again, you may need to consider this when joining reports.
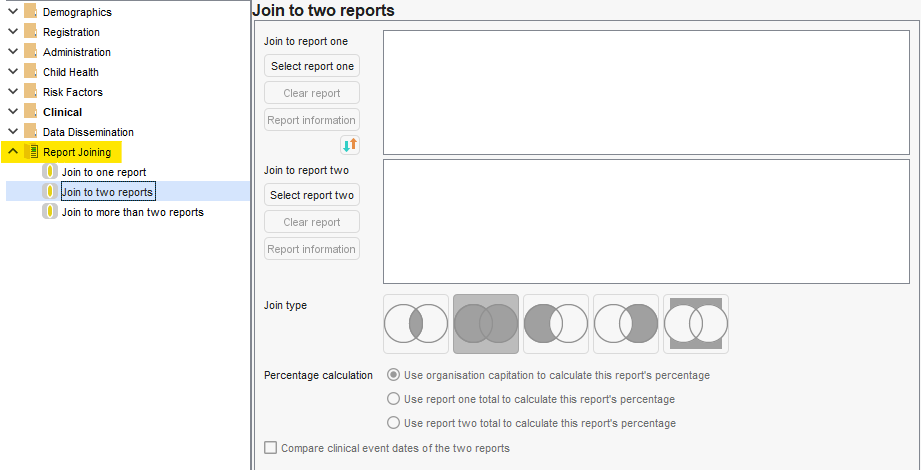
Report joining
Many tools (including where staff are trained by colleagues) only tend to demonstrate joining reports that are in the same category by selecitng 2+ reports and clicking the 'Join' button the menu. This can be convenient, but will significantly restrict your reporting joining options if there isn't awareness of other ways.
Reporting Joining is always available when creating/amending a report it is the last category. You can join to one, two or 3+ reports.
Using reports from elsewhere
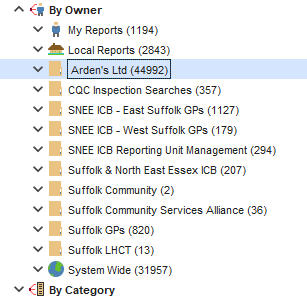
If you have access to reports owned by other services, these can be run or integrated (joined) into your own reports.
There are two types:
- System Wide (globe icon - nationally published by TPP, includes QOF and ES reports etc)
- Organisation Groups (yellow folders e.g. Ardens)
- If you join to published reports, you will benefit from any automatic updates from the publishing organisation during the lifecycle of that report - however, many centrally-published reports have a maximum lifecycle of one year. Others (such as for enhanced services) have dates that change at the national extraction intervals (monthly/quarterly etc).
- Where joining to reports such as QOF, if you intend to use them beyond the current QOF year you will need to review and update your reports joining to the latest versions. If you need to use them prior to the release of the latest version, you may need to build you own to ensure you can provide care during this period.
- If your organisation copies published reports, you will not automatically receive updates/corrections through the lifecycle of the published report and are now indefinitely responsible for managing those reports, including routinely reviewing to ensure they remain clinically safe/appropriate.
Was this article helpful?
That’s Great!
Thank you for your feedback
Sorry! We couldn't be helpful
Thank you for your feedback
Feedback sent
We appreciate your effort and will try to fix the article